Chemistry, 08.07.2021 21:50 jjiopppotdd5638
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction spontaneous?
A. At no temperature
B. T< 50 K
C. T>617 K
D. T< 617 K

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 06:30, amylumey2005
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
For a reaction, AH = 176 kJ/mol and A SO = 0.285 kJ/(K•mol). At what
temperatures is this reaction...
Questions in other subjects:


Mathematics, 11.03.2021 20:50

Mathematics, 11.03.2021 20:50

Mathematics, 11.03.2021 20:50

Mathematics, 11.03.2021 20:50



Mathematics, 11.03.2021 20:50

Mathematics, 11.03.2021 20:50

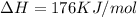
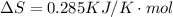
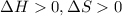
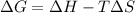
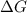 is negative, then the reaction is spontaneous.
is negative, then the reaction is spontaneous.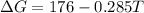
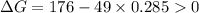
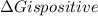 .Hence, the reaction is not spontaneous.
.Hence, the reaction is not spontaneous.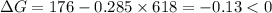
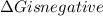 .Hence, the reaction is spontaneous.
.Hence, the reaction is spontaneous.