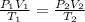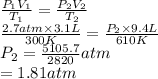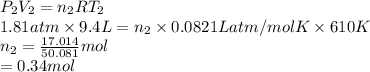A cylinder contains 3.1 L of oxygen at 300 K and 2.7 atm. The gas is heated, causing a piston in the cylinder to move outward. The heating causes the temperature to rise to 610 K and the volume of the cylinder to increase to 9.4 L.
How many moles of gas are in the cylinder?
Express your answer using two significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, jescanarias22
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A cylinder contains 3.1 L of oxygen at 300 K and 2.7 atm. The gas is heated, causing a piston in the...
Questions in other subjects:




Mathematics, 27.12.2019 06:31

History, 27.12.2019 06:31

History, 27.12.2019 06:31


English, 27.12.2019 06:31

Mathematics, 27.12.2019 06:31

English, 27.12.2019 06:31

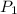 = 2.7 atm,
= 2.7 atm, 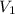 = 3.1 L,
= 3.1 L, 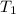 = 300 K
= 300 K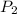 = ?,
= ?, 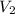 = 9.4 L,
= 9.4 L, 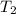 = 610 K
= 610 K