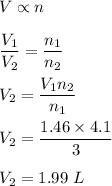Chemistry, 08.07.2021 04:40 homeschool0123
A reaction produces 3.0 mol of gas, which occupies 1.46 L. What is the volume of the product when 4.1 mol are produced at constant temperature and pressure?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
A reaction produces 3.0 mol of gas, which occupies 1.46 L. What is the volume of the product when 4....
Questions in other subjects:

Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00

Biology, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00