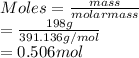Chemistry, 07.07.2021 20:20 crawford184232323234
Calculate the molarity of 198 g of barium iodide (Bal2) in 2.0 l of solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Calculate the molarity of 198 g of barium iodide (Bal2) in 2.0 l of solution...
Questions in other subjects:

World Languages, 01.06.2021 06:20

History, 01.06.2021 06:20

Computers and Technology, 01.06.2021 06:20


Social Studies, 01.06.2021 06:20

Mathematics, 01.06.2021 06:20



Biology, 01.06.2021 06:30

 in 2.0 L of solution is 0.253 M.
in 2.0 L of solution is 0.253 M.