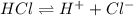Chemistry, 07.07.2021 01:00 laytonlutz
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substance that . increases Ka when placed in H2O increases [OH-] when placed in H2O acts as a proton donor acts as a proton acceptor decreases [H ] when placed in H2O

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


You know the right answer?
A Bronsted-Lowry acid is defined as a substance that . A Bronsted-Lowry acid is defined as a substan...
Questions in other subjects:




Mathematics, 21.08.2020 17:01





Health, 21.08.2020 17:01