Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1
You know the right answer?
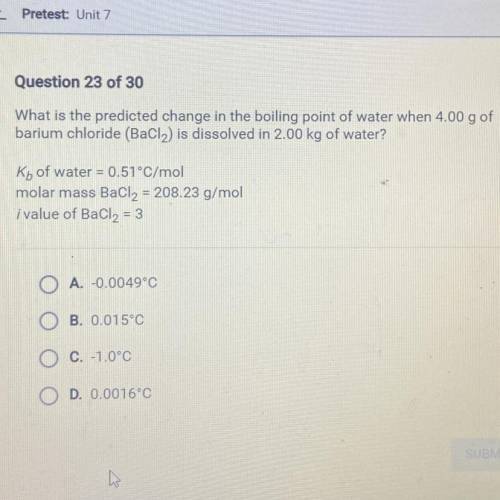
What is the predicted change in the boiling point of water when 4.00 g of
barium chloride (BaCl2) i...
Questions in other subjects:



Mathematics, 06.05.2020 02:33


Social Studies, 06.05.2020 02:33


English, 06.05.2020 02:33

Mathematics, 06.05.2020 02:33


History, 06.05.2020 02:33