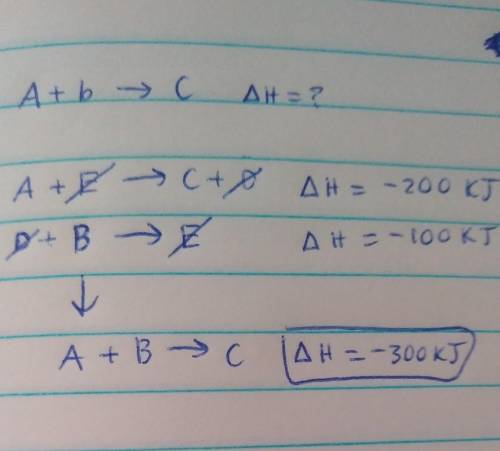Chemistry, 05.07.2021 23:20 anavallesdemiguel2
Determine the enthalpy for the reaction A+B --> C If we know the following:
A+E --> C+D; delta H = -200 kJ
D+B --> E; delta H = -100 kJ

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Determine the enthalpy for the reaction A+B --> C If we know the following:
A+E --> C+D; delt...
Questions in other subjects:


English, 09.03.2021 17:40



English, 09.03.2021 17:40

Mathematics, 09.03.2021 17:40




Mathematics, 09.03.2021 17:40