

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, shafferakr6
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

You know the right answer?
Decide if the following statements are true or false.
a. Ca has a larger ionization energy than Ca2...
Questions in other subjects:


Mathematics, 13.07.2020 19:01

Social Studies, 13.07.2020 19:01

Mathematics, 13.07.2020 19:01





Mathematics, 13.07.2020 19:01

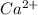 - False
- False
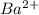 - False
- False
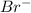 - True
- True
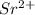 is larger than Sr - False
is larger than Sr - False


