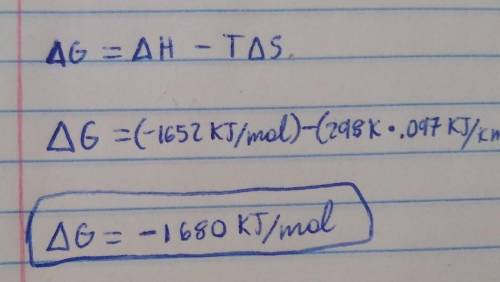Chemistry, 03.07.2021 01:20 laura52677
At 298 K, AHO = -1652 kJ/mol and ASO = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
At 298 K, AHO = -1652 kJ/mol and ASO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the react...
Questions in other subjects:

Mathematics, 08.12.2021 14:00



Arts, 08.12.2021 14:00

Mathematics, 08.12.2021 14:00


English, 08.12.2021 14:00