Crystals of sodium chloride were prepared by the following method.
1 25.0 cm3
of dilute...

Chemistry, 02.07.2021 17:20 daltonrebekah3440
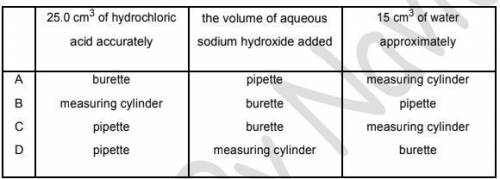
Crystals of sodium chloride were prepared by the following method.
1 25.0 cm3
of dilute hydrochloric acid was accurately measured into a conical flask.
2 Aqueous sodium hydroxide was added until the solution was neutral. The volume of
sodium hydroxide added was measured.
3 The solution was evaporated and the crystals washed with approximately 15 cm3
of water.
Which row shows the pieces of apparatus used to measure the 25.0 cm3
of hydrochloric acid, the
volume of aqueous sodium hydroxide and the 15 cm3
of water?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Questions in other subjects:




Health, 14.09.2019 08:20

Biology, 14.09.2019 08:20


History, 14.09.2019 08:20

Health, 14.09.2019 08:20

History, 14.09.2019 08:20



