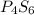Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3



Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Compound X has a molar mass of 316.25 g*mol^-1 and the following composition:
element & mass %<...
Questions in other subjects:


Arts, 09.11.2020 18:40

Computers and Technology, 09.11.2020 18:40

Geography, 09.11.2020 18:40


Biology, 09.11.2020 18:40


Mathematics, 09.11.2020 18:40

History, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40