

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
You know the right answer?
how many distinct monochlorinated products can result when methylcyclohexane is subjected to free ra...
Questions in other subjects:

English, 22.01.2021 21:40






Mathematics, 22.01.2021 21:40

English, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40

Mathematics, 22.01.2021 21:40

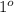 , secondary
, secondary  and tertiary
and tertiary 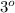 carbons as shown in the image.
carbons as shown in the image.



