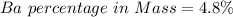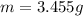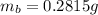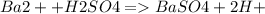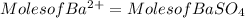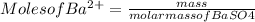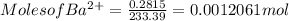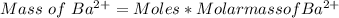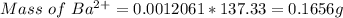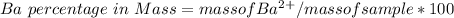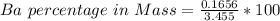Chemistry, 01.07.2021 04:20 mucciak2854
3. A 3.455-g sample of a mixture was analyzed for barium ion by adding a small excess of sulfuric acid to an aqueous solution of the sample. The resultant reaction produced a precipitate of barium sulfate, which was collected by filtration, washed, dried, and weighed. If 0.2815 g of barium sulfate was obtained, what was the mass percentage of barium in the sample

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
3. A 3.455-g sample of a mixture was analyzed for barium ion by adding a small excess of sulfuric ac...
Questions in other subjects:

History, 25.02.2021 03:20

Computers and Technology, 25.02.2021 03:20


Social Studies, 25.02.2021 03:20


Spanish, 25.02.2021 03:20


English, 25.02.2021 03:20

Mathematics, 25.02.2021 03:20