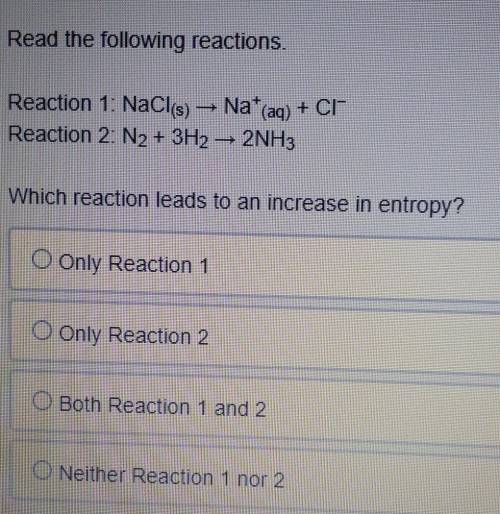
Read the following reactions.
Reaction 1: NaCl(s) -> Na+ (aq) + CI-
Reaction 2: N2 + 3H2 -&...


Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Questions in other subjects: