
Chemistry, 29.06.2021 19:00 reagancunningham2004
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl3 (g) + Cl2 (g)
A vessel is charged with PCl 5 giving an initial pressure of 0.123 atm and yields PCl 3 and Cl 2. At equilibrium, the partial pressure of PCl 3 is atm.
A) 0.0782.
B) 0.0455.
C) 0.0908.
D) 0.0330.
E) 0.123.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


You know the right answer?
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl...
Questions in other subjects:

Mathematics, 06.11.2020 23:20

Mathematics, 06.11.2020 23:20

History, 06.11.2020 23:20

Mathematics, 06.11.2020 23:20

History, 06.11.2020 23:20


Mathematics, 06.11.2020 23:20


Mathematics, 06.11.2020 23:20

Computers and Technology, 06.11.2020 23:20

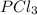 is 0.0330 atm.
is 0.0330 atm.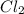 . Hence, let us assume that x quantity of
. Hence, let us assume that x quantity of 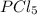 is decomposed and gives x quantity of
is decomposed and gives x quantity of 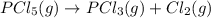
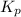 of this reaction is as follows.
of this reaction is as follows.![K_{p} = \frac{[PCl_{3}][Cl_{2}]}{[PCl_{5}]}\\0.0121 = \frac{x \times x}{(0.123 - x)}\\x = 0.0330](/tpl/images/1386/0346/b037d.png)


