b) 15g NO

Chemistry, 29.06.2021 14:50 devin030505
Identify the substance which has same number of molecules in 14g N2
a) 3.4g NH3
b) 15g NO
c)64g O2
d) 32g SO2
e)1g H2
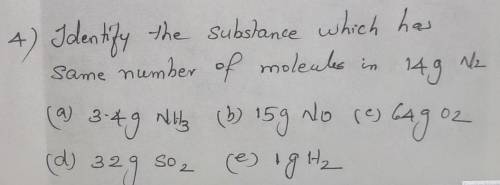

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Identify the substance which has same number of molecules in 14g N2
a) 3.4g NH3
b) 15g NO
b) 15g NO
Questions in other subjects:





Mathematics, 26.08.2019 21:40

Health, 26.08.2019 21:40

Mathematics, 26.08.2019 21:40

Mathematics, 26.08.2019 21:40


History, 26.08.2019 21:40



