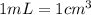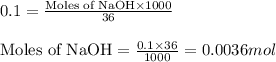Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Calculate the number of moles of NaOH in an aqueous solution of 36 cm3 of 0.1 mol dm−3.
PLSS EXPLAI...
Questions in other subjects:


History, 08.11.2019 14:31


History, 08.11.2019 14:31



Mathematics, 08.11.2019 14:31

Mathematics, 08.11.2019 14:31

History, 08.11.2019 14:31

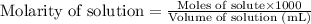 .....(1)
.....(1)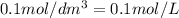 (Conversion factor:
(Conversion factor: 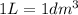
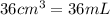 (Conversion factor:
(Conversion factor: