Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
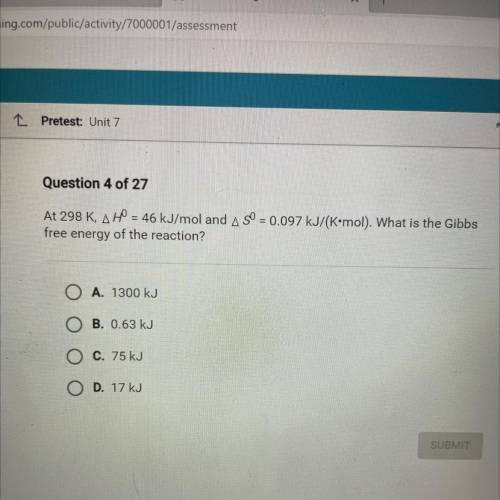
At 298 K, AH = 46 kJ/mol and A SO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the reaction...
Questions in other subjects:

Chemistry, 24.02.2021 18:00

Mathematics, 24.02.2021 18:00


Mathematics, 24.02.2021 18:00

History, 24.02.2021 18:00

Mathematics, 24.02.2021 18:00

Mathematics, 24.02.2021 18:00