
Chemistry, 24.06.2021 22:00 aryannaholmes9
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydrogen atom.
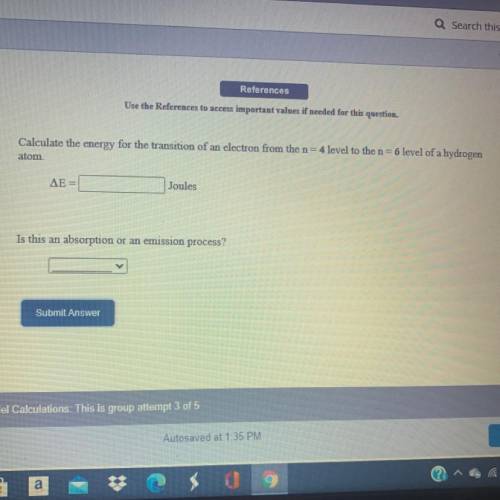

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 23.06.2019 07:30, Ayyyyeeeeeeewuzgud
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydr...
Questions in other subjects:


Mathematics, 06.03.2020 02:24



Geography, 06.03.2020 02:24




Mathematics, 06.03.2020 02:24




