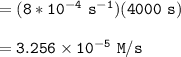The decomposition of hydrogen peroxide was studied, and the following data were obtained at a particular temperature. Time (s)[H2O2] (mol/L)0 1.00120 ± 10.91300 ± 10.78600 ± 10.591200 ± 10.371800 ± 10.222400 ± 10.133000 ± 10.0823600 ± 10.050Assuming that the rate= -delta [H2O2]/delta t determine the rate law, integrated rate law, and the value of the rate constant. Calculate [H2O2] at 4000. s after the start of the reaction.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
The decomposition of hydrogen peroxide was studied, and the following data were obtained at a partic...
Questions in other subjects:


Mathematics, 06.12.2020 01:00


History, 06.12.2020 01:00

German, 06.12.2020 01:00

Mathematics, 06.12.2020 01:00




 which declines exponentially in relation to the time and it obeys the equation:
which declines exponentially in relation to the time and it obeys the equation: 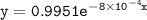
![\mathtt{ [A] = [A]_o e^{-kt}}](/tpl/images/1383/5053/858f7.png)

![\mathtt{= k[H_2O_2]}](/tpl/images/1383/5053/b66a7.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-(8*10^{-4})t}}](/tpl/images/1383/5053/3f9ba.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-8*10^{-4}(t)}}](/tpl/images/1383/5053/adf3d.png)
![\mathtt{[H_2O_2] = (1.00\ M)*e^{-8*10^{-4}(4000)\ s}}](/tpl/images/1383/5053/a221b.png)
![\mathtt{[H_2O_2] =0.0407 \ M}](/tpl/images/1383/5053/c14b9.png)