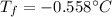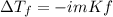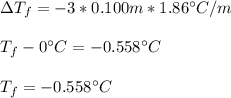Chemistry, 24.06.2021 20:20 daebreonnakelly
Calculate the freezing point of a 0.100 m aqueous solution of K2SO4, taking interionic attractions into consideration by using the van't Hoff factor (i for 0.100 m K2SO4

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Calculate the freezing point of a 0.100 m aqueous solution of K2SO4, taking interionic attractions i...
Questions in other subjects:

Mathematics, 10.03.2021 21:50


Mathematics, 10.03.2021 21:50

Mathematics, 10.03.2021 21:50

Mathematics, 10.03.2021 21:50

History, 10.03.2021 21:50

Computers and Technology, 10.03.2021 21:50

Mathematics, 10.03.2021 21:50


Mathematics, 10.03.2021 21:50