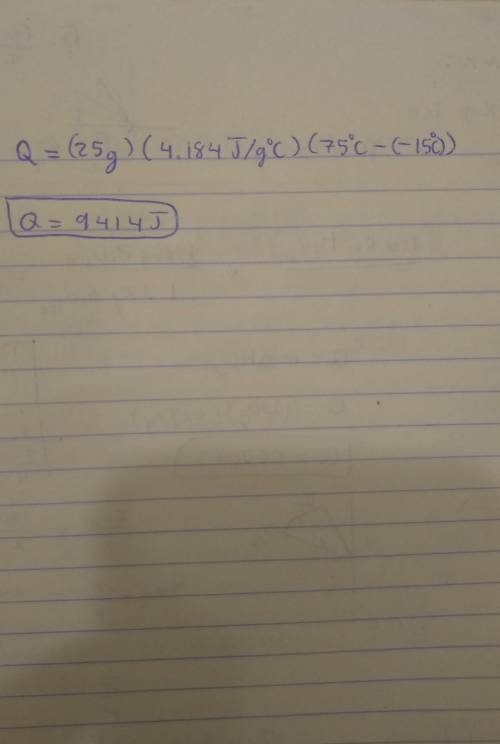Chemistry, 24.06.2021 03:30 sevaramirabell
Note: Please show all work and calculation setups to get full credit. T. he following may be used on this assignment: specific heat of (water=4.184 J/g oC; ice=2.03 J/g oC; steam=1.99 184 J/g oC); heat of fusion of water=80. cal/g; heat of vaporization=540 cal/g; 1cal=4.184J. Calculate the energy required (in J) to convert 25 g of ice at -15 oC to water at 75 oC.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Note: Please show all work and calculation setups to get full credit. T. he following may be used on...
Questions in other subjects:

Mathematics, 30.10.2020 21:40



Mathematics, 30.10.2020 21:40

Social Studies, 30.10.2020 21:40