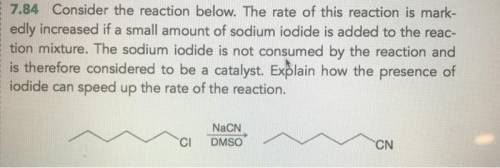
Chemistry, 23.06.2021 01:50 karennayeli
The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and is therefore considered to be a catalyst. Explain how the presence of iodide can speed up the rate of the reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the r...
Questions in other subjects:



History, 17.09.2019 08:30

Social Studies, 17.09.2019 08:30


Mathematics, 17.09.2019 08:50

Physics, 17.09.2019 08:50

Mathematics, 17.09.2019 08:50

Social Studies, 17.09.2019 08:50

English, 17.09.2019 08:50