Buffer Calculation Questions
um Question 14 ( points)
Calculate the pH of a buffer that conta...

Chemistry, 22.06.2021 17:10 lilyrockstarmag
Buffer Calculation Questions
um Question 14 ( points)
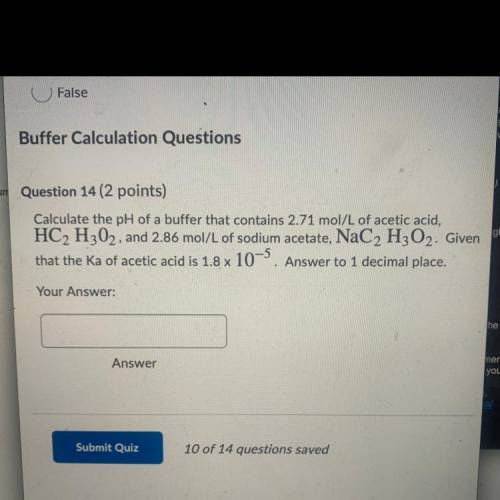
Calculate the pH of a buffer that contains 2.71 mol/L of acetic acid,
HC2 H302, and 2.86 mol/L of sodium acetate, NaC2 H2O2. Given
that the Ka of acetic acid is 1.8 x 10-5. Answer to 1 decimal place.
Your
Answer

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Questions in other subjects:












