
Chemistry, 22.06.2021 04:40 bearminar2156
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when 113.6 grams of CuO are consumed?
Round your answer to the nearest tenth. If your answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect. :
Element Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
In the above equation, how many moles of N2 can be made when...
Questions in other subjects:



Mathematics, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00


Health, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Chemistry, 01.12.2020 01:00

 are produced in the reaction
are produced in the reaction ......(1)
......(1) = 113.6 g
= 113.6 g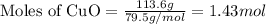

 of
of 

