Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 09:00, rebeccathecatt
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
You know the right answer?
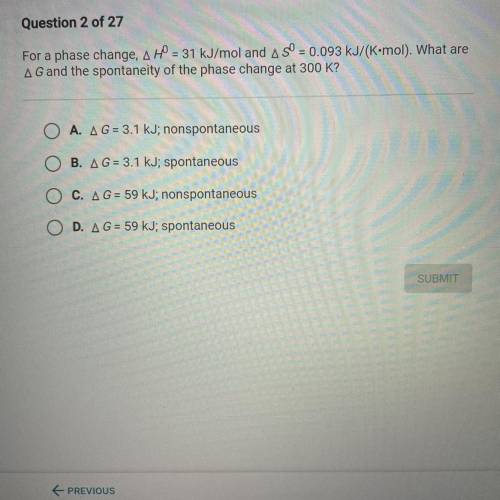
For a phase change, A H0 = 31 kJ/mol and A S0 = 0.093 kJ/(K•mol). What are
A G and the spontaneity...
Questions in other subjects:

English, 27.10.2020 01:00

Computers and Technology, 27.10.2020 01:00

History, 27.10.2020 01:00

History, 27.10.2020 01:00



History, 27.10.2020 01:00

Biology, 27.10.2020 01:00

Chemistry, 27.10.2020 01:00