gas

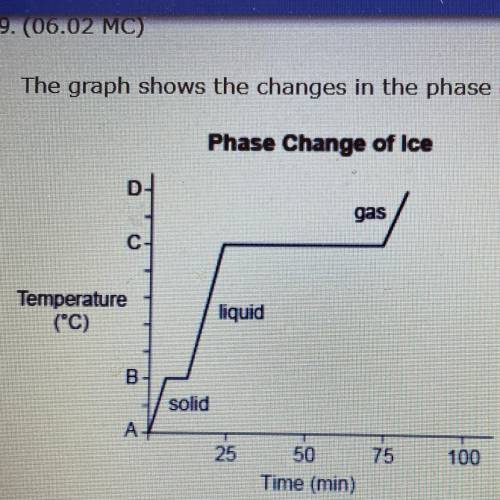
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
C
Temperature
(C)
liquid
в.
solid
A-
25
50
75
100
Time (min)
Which of the following temperatures describes the value of A? (5 points)
0 °C, because it is the melting point gf ice.
O 0 °C, because it is the freezing point of water.
Less than 0 °C, because B represents the temperature at which ice melts.
Less than 0 °C, because B represents the temperature at which water evaporates.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
The graph shows the changes in the phase of ice when it is heated.
Phase Change of Ice
gas
gas
Questions in other subjects:

Social Studies, 02.03.2021 19:10





Mathematics, 02.03.2021 19:10

Social Studies, 02.03.2021 19:10


Mathematics, 02.03.2021 19:10



