
Chemistry, 20.06.2021 20:30 sillslola816oxb5h7
A 6.00 g sample of an optically pure compound was dissolved in 40.0 mL of CCl4. The observed rotation was +3.30 °, measured in a 10.0 cm (1.00 dm) polarimeter tube.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 21:30, jessicaisbaehood
Enzymes are always consumed by catalyzing a reaction increase the rate at which a reaction occurs sometimes increase the amount of energy necessary to initiate a reaction catalyze reactions that release energy, but not those that consume energy
Answers: 1

Chemistry, 23.06.2019 23:00, kitttimothy55
Putting maximum amount of points a solution is made by dissolving 10.20 grams of glucose (c6h12o6) in 355 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1
You know the right answer?
A 6.00 g sample of an optically pure compound was dissolved in 40.0 mL of CCl4. The observed rotatio...
Questions in other subjects:



Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00



History, 09.04.2021 01:00

Social Studies, 09.04.2021 01:00


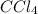 and the observed rotation was +3.30°, measured in a 10.0 cm (1.00 dm) polarimeter tube, how would one determine the specific rotation of the pure compound?
and the observed rotation was +3.30°, measured in a 10.0 cm (1.00 dm) polarimeter tube, how would one determine the specific rotation of the pure compound?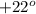
![[\alpha]=\frac{\alpha_{\text{observed}}}{C\times l\text{( in dm)}}](/tpl/images/1379/8967/197aa.png)
![[\alpha]](/tpl/images/1379/8967/b7f66.png) = specific rotation of a pure compound
= specific rotation of a pure compound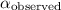 = observed rotation of the compound = [ex]+3.30^o[/tex]
= observed rotation of the compound = [ex]+3.30^o[/tex]![[\alpha]=\frac{+3.30^o}{0.15\times 1.0}](/tpl/images/1379/8967/6befd.png)
![[\alpha]=+22^o](/tpl/images/1379/8967/7008b.png)


