
Chemistry, 19.06.2021 18:10 pattykline
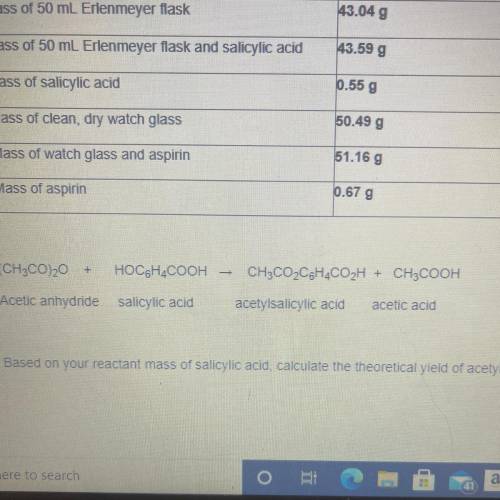
Based on your reactant mass of salicylic acid calculate the theoretical trio of acetysalicylic acid
The chart is in the picture
Please help I’ll give you brainiest

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 04:20, lelliott86
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
Based on your reactant mass of salicylic acid calculate the theoretical trio of acetysalicylic acid...
Questions in other subjects:


Mathematics, 16.12.2020 21:20

English, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20

Mathematics, 16.12.2020 21:20


Mathematics, 16.12.2020 21:20

English, 16.12.2020 21:20



