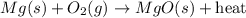Chemistry, 18.06.2021 07:40 adamkinney6110
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as shown in the following unbalanced equation.
Mg (s) + O2 (g) → MgO (s) + heat
What two types of reactions could this chemical equation be classified as?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 23:00, ashyding4800
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as...
Questions in other subjects:


Mathematics, 12.01.2021 17:40

Mathematics, 12.01.2021 17:40




Physics, 12.01.2021 17:40