Chemistry, 17.06.2021 21:50 clairebear65
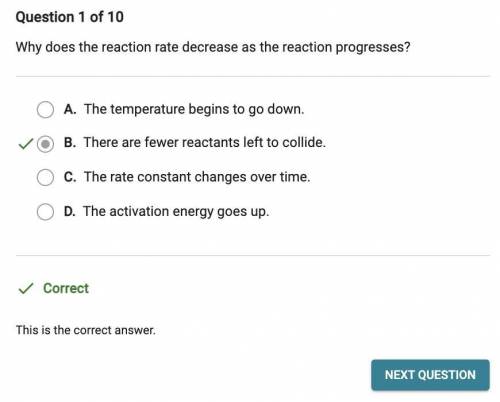
Why does the reaction rate decrease as the reaction progresses?
O A. The temperature begins to go down.
B. The activation energy goes up.
O C. The rate constant changes over time.
O D. There are fewer reactants left to collide.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Why does the reaction rate decrease as the reaction progresses?
O A. The temperature begins to go d...
Questions in other subjects:

Mathematics, 05.05.2020 22:29




Geography, 05.05.2020 22:29


Biology, 05.05.2020 22:29

Mathematics, 05.05.2020 22:29

World Languages, 05.05.2020 22:29