
Chemistry, 17.06.2021 16:20 juanitarodrigue
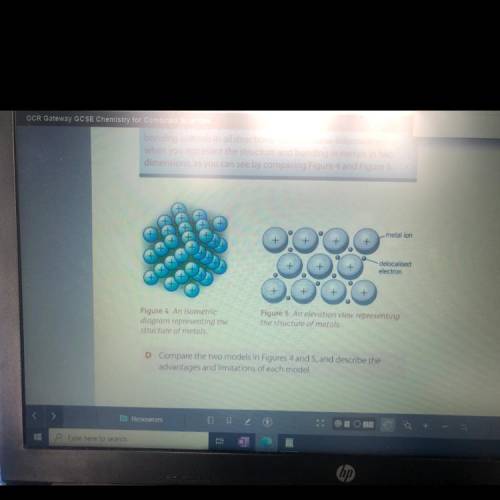
Some students who looked at Figures 4 and 5 did not realise
that they applied to all metals. Instead, they thought that the
figures only showed a metal from Group 1 of the Periodic Table.
Suggest why they thought this, and describe changes the students
might have made so that the diagrams showed a metal from
Group 2
(5 marks)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Some students who looked at Figures 4 and 5 did not realise
that they applied to all metals. Instea...
Questions in other subjects:

Mathematics, 10.10.2019 15:10


Mathematics, 10.10.2019 15:10

History, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10

Mathematics, 10.10.2019 15:10




Mathematics, 10.10.2019 15:10



