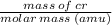Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
One atom of chromium (Cr) has a mass of 52.00 amu. How many chromium atoms does it take to equal a m...
Questions in other subjects:

Mathematics, 27.02.2021 17:00

Mathematics, 27.02.2021 17:00

Mathematics, 27.02.2021 17:00

Mathematics, 27.02.2021 17:00


Computers and Technology, 27.02.2021 17:00

Law, 27.02.2021 17:00

Mathematics, 27.02.2021 17:00

Social Studies, 27.02.2021 17:00