
Chemistry, 16.06.2021 22:20 erica11223344
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl2 (aq)
OA. K=[NO_ Cl]?[NO2]?[ciz]
[NO_C1]
[NO2]?[Ciz]
OB, K=
Ock=
2[NO_IC12]
2[NO2C]
OD. K=
2[NO2Cl]
2[NO2][Ciz]
OEK=
[NO2][C12]
[NO_C1]?
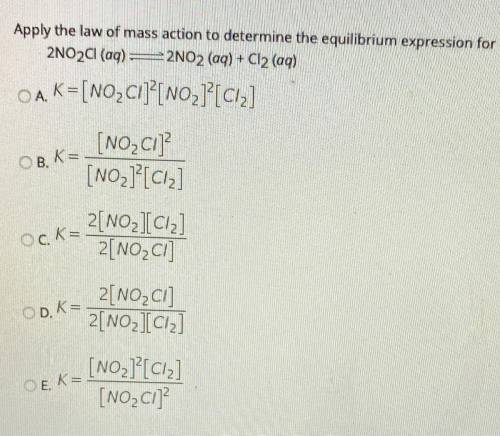

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl...
Questions in other subjects:


Chemistry, 19.05.2020 15:15










