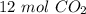Chemistry, 16.06.2021 20:40 dylanjones6996
6CO2 + 6H20 --> C6H12O6 + 602 What is the total number of moles of CO2 needed to make 2 moles of CH1206?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
6CO2 + 6H20 --> C6H12O6 + 602
What is the total number of moles of CO2 needed to make 2 moles of...
Questions in other subjects:






Mathematics, 09.06.2021 21:30

Mathematics, 09.06.2021 21:30

English, 09.06.2021 21:30

Business, 09.06.2021 21:30

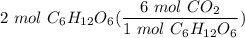 [DA] Multiply [Cancel out units]:
[DA] Multiply [Cancel out units]: