Chemistry, 15.06.2021 20:30 lanettejohnson355
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solution is 150 mL. What volume of a 1.25 M solution could be made from the stock solution?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 15:50, nagwaelbadawi
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 2
You know the right answer?
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solutio...
Questions in other subjects:





English, 17.04.2021 17:20




Mathematics, 17.04.2021 17:20

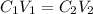 ....(1)
....(1)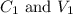 are the concentration and volume of stock solution.
are the concentration and volume of stock solution.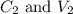 are the concentration and volume of diluted solution.
are the concentration and volume of diluted solution.