
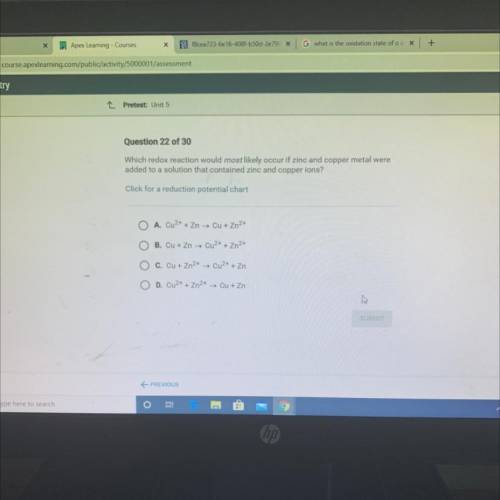
Chemistry, 15.06.2021 18:20 aliciabenitez
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that contained zinc and copper ions?
Click for a reduction potential chart
A. Cu2+ + Zn → Cu + Zn2+
O B. Cu + Zn → Cu2+ + Zn2+
C. Cu + Zn2+ → Cu2+ + Zn
O D. Cu2+ + Zn2+ → Cu + Zn

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that...
Questions in other subjects:

Spanish, 13.11.2019 12:31


Mathematics, 13.11.2019 12:31





Mathematics, 13.11.2019 12:31

Mathematics, 13.11.2019 12:31




