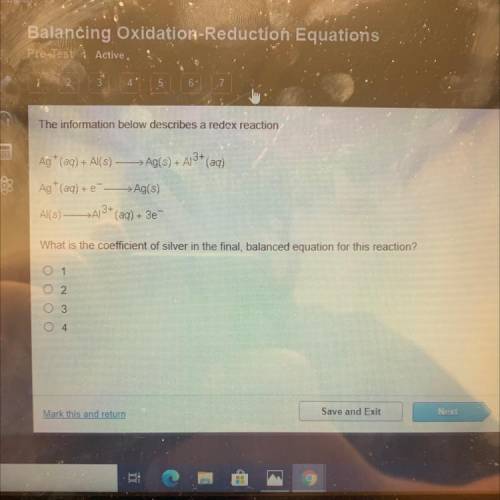
The information below describes a redox reaction.
Ag+ (aq) + Al(s) —>Ag(s) + A13+ (aq)
Ag+...

Chemistry, 15.06.2021 03:50 rubyhart522
The information below describes a redox reaction.
Ag+ (aq) + Al(s) —>Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s) - >A3+ (aq) + 3e
What is the coefficient of silver in the final, balanced equation for this reaction?
0 1
02
0 3
O 4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

You know the right answer?
Questions in other subjects:




Mathematics, 09.09.2021 05:10


Mathematics, 09.09.2021 05:10


Biology, 09.09.2021 05:10


Mathematics, 09.09.2021 05:10





