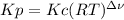Chemistry, 14.06.2021 17:00 therealrg10
Equilibrium constants for gases can be expressed in terms of concentrations, Kc, or in terms of partial pressures, Kp. Which one of the following statements regarding Kc and Kp is correct?
a. Kc and Kp are equal when all stoichiometric coefficients in the balanced reaction equation equal one.
b. Kc and Kp are equal when the conditions are standard (P= 1 atm, T=298 K)
c. Kc and Kp are equal when the sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
d. Kc and Kp can never be equal.
e. Kc and Kp have the same values but different units.

Answers: 1


Other questions on the subject: Chemistry




You know the right answer?
Equilibrium constants for gases can be expressed in terms of concentrations, Kc, or in terms of part...
Questions in other subjects:

Chemistry, 21.02.2020 23:08




Mathematics, 21.02.2020 23:08

Mathematics, 21.02.2020 23:08