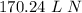What volume will 106.476 g N, gas at STP occupy?
O 85.1808L
O 170.362L
O 7.60543L
...

Chemistry, 13.06.2021 05:40 pg67891012345
What volume will 106.476 g N, gas at STP occupy?
O 85.1808L
O 170.362L
O 7.60543L
0 3.80271L

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 22:00, darkremnant14
2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample. show your work. b. calculate the moles of each element in c12h22o11, show your work c. calculate the number of atoms of each type in c12h22o11. show your work
Answers: 2

Chemistry, 24.06.2019 00:00, lola06032003
Sodium azide is a substance that can be used to inflate airbags. an electrical impulse causes the sodium azide to decompose, producing elemental sodium and nirtogen gas. write the balanced chemical equation for this reaction. plzzz
Answers: 1
You know the right answer?
Questions in other subjects:




History, 17.09.2021 04:20

Mathematics, 17.09.2021 04:20


Mathematics, 17.09.2021 04:20


Health, 17.09.2021 04:20

Biology, 17.09.2021 04:20

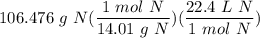 [DA] Divide/Multiply [Cancel out units]:
[DA] Divide/Multiply [Cancel out units]: