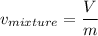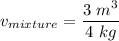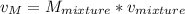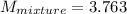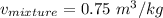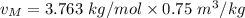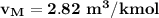Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
2 kg of hydrogen (H2) is mixed with 2 kg of oxygen (O2). If the final mixture has a volume of 3 m3:...
Questions in other subjects:

Arts, 10.10.2021 09:20


Chemistry, 10.10.2021 09:20

English, 10.10.2021 09:20

Mathematics, 10.10.2021 09:20

Biology, 10.10.2021 09:20


Mathematics, 10.10.2021 09:20

Mathematics, 10.10.2021 09:20