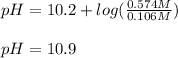Chemistry, 12.06.2021 02:30 addisonwiles
A buffer solution contains 0.496 M KHCO3 and 0.340 M K2CO3. If 0.0585 moles of potassium hydroxide are added to 250. mL of this buffer, what is the pH of the resulting solution

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

You know the right answer?
A buffer solution contains 0.496 M KHCO3 and 0.340 M K2CO3. If 0.0585 moles of potassium hydroxide a...
Questions in other subjects:

Social Studies, 24.06.2019 01:00

Biology, 24.06.2019 01:00


Mathematics, 24.06.2019 01:00


History, 24.06.2019 01:00

History, 24.06.2019 01:00




![[CO_3^{2-}]=\frac{0.1435mol}{0.25L}=0.574M \\](/tpl/images/1372/2885/db894.png)
![[HCO_3^{-}]=\frac{0.0265mol}{0.25L}=0.106M](/tpl/images/1372/2885/2de97.png)