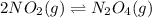Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

You know the right answer?
What happens to the equilibrium between NO2(g) and N2O4(g) in inert argon when the volume is increas...
Questions in other subjects:






Mathematics, 15.11.2019 04:31


Mathematics, 15.11.2019 04:31