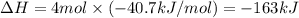Chemistry, 11.06.2021 06:50 smcardenas02
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY need.
specific heats heat of fusion heat of vaporization
H2O(s) = 2.1 J/g0C 6.01 kJ/mole 40.7 kJ/mole
H2O(L) = 4.18 J/g0C
H2O(g) = 1.7 J/g0C
2930 kJ
163 kJ
-163 kJ
-2930 kJ

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
What is the delta H when 72.0 grams H2O condenses at 100.00C?
Here are some constants that you MAY...
Questions in other subjects:

Mathematics, 17.10.2019 17:00


History, 17.10.2019 17:00

Biology, 17.10.2019 17:00


Chemistry, 17.10.2019 17:00


English, 17.10.2019 17:00

History, 17.10.2019 17:00

 is -163 kJZ
is -163 kJZ ......(1)
......(1)
 ......(2)
......(2) = specific heat of vaporization = -40.7 kJ/mol
= specific heat of vaporization = -40.7 kJ/mol