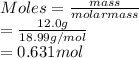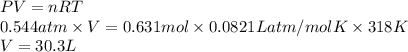Chemistry, 11.06.2021 06:50 bgallman153p71edg
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume of the container?
R = 0.0821 L atm/mol K
15.1 L
4.29 L
30.3 L
2.14 L

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, amanda2003teddy
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3


Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume...
Questions in other subjects:




Physics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

History, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

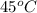 = (45 + 273) K = 318 K
= (45 + 273) K = 318 K