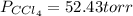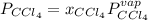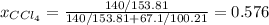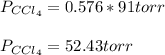At a certain temperature the vapor pressure of pure chloroform (CHCl3) is measured to be 91. torr. Suppose a solution is prepared by mixing 140. g of chloroform and 67.1 g of heptane (C, H16) of chloroform and 67.1 g of heptane (C7H16 Calculate the partial pressure of chloroform vapor above this solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
At a certain temperature the vapor pressure of pure chloroform (CHCl3) is measured to be 91. torr. S...
Questions in other subjects:



Business, 14.06.2021 17:10

Mathematics, 14.06.2021 17:10



Spanish, 14.06.2021 17:10