
Chemistry, 10.06.2021 21:50 Imamdiallo18
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g of Cl2 react?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Given the equation 2Si(s) + 2Cl2(g) --> 2SiCl2(g) + 687 kJ, how much heat is produced when 106 g...
Questions in other subjects:


Mathematics, 08.03.2021 23:20


Mathematics, 08.03.2021 23:20



Mathematics, 08.03.2021 23:20

Mathematics, 08.03.2021 23:20


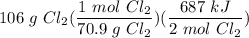 [DA] Divide/Multiply [Cancel out units]:
[DA] Divide/Multiply [Cancel out units]: 


