
Chemistry, 10.06.2021 21:30 xxgissellexx
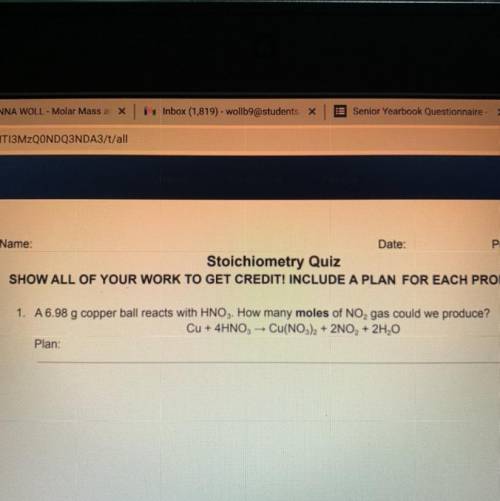
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce? Cu + 4HNO3 → Cu(NO3)2 + 2NO, + 2H2O

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
A 6.98 g copper ball reacts with HNO3. How many moles of NO, gas could we produce?
Cu + 4HNO3 → Cu(...
Questions in other subjects:



Mathematics, 22.01.2021 23:20

Mathematics, 22.01.2021 23:20

English, 22.01.2021 23:20

Business, 22.01.2021 23:20

Mathematics, 22.01.2021 23:20

Biology, 22.01.2021 23:20




