8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate<...

Chemistry, 10.06.2021 20:30 giusto1073
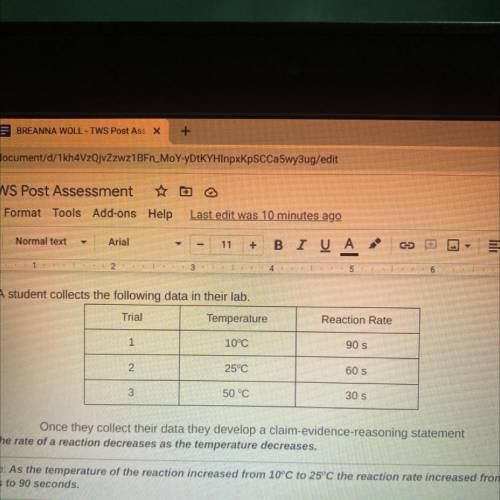
8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate
Once they collect their data they develop a claim-evidence-reasoning statement
Claim: The rate of a reaction decreases as the temperature decreases.
Evidence: As the temperature of the reaction increased from 10°C to 25°C the reaction rate increased from 60
seconds to 90 seconds.
Reasoning: Reaction rate depends on the temperature of the reactants. So when the temperature decreases, so
should the reaction rate. Particles need to collide in order to react. Lower temperatures allow molecules to move
slow enough to stick together and react so the reaction rate decreases.
Use the space below to provide feedback to the student. Was their conclusion correct? How should they fix it?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
Questions in other subjects:

History, 30.08.2019 13:30

History, 30.08.2019 13:30

Computers and Technology, 30.08.2019 13:30

History, 30.08.2019 13:30


Mathematics, 30.08.2019 13:30


Arts, 30.08.2019 13:30

History, 30.08.2019 13:30

Mathematics, 30.08.2019 13:30



