
Chemistry, 10.06.2021 14:10 sherlock19
The Copper Chloride solution used in the investigation contained 300 grams per dm3 of solid CuCl2 dissolved in 1dm3 of water. The student used 50cm3 of copper chloride solution in each experiment. Calculate the mass of solid copper chloride used in each experiment.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 10:10, estebanmff
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3
You know the right answer?
The Copper Chloride solution used in the investigation contained 300 grams per dm3 of solid CuCl2 di...
Questions in other subjects:

Biology, 07.10.2019 04:30

Biology, 07.10.2019 04:30


History, 07.10.2019 04:30


Advanced Placement (AP), 07.10.2019 04:30

Mathematics, 07.10.2019 04:30

English, 07.10.2019 04:30


Mathematics, 07.10.2019 04:30

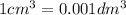
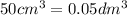
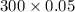 g
g

